Got wrong the valence electrons in Rb are in a higher energy level than those of K or the outer orbitals have a higher principal quantum number in rb than in K or the valence electrons of Rb are less attracted to the nucleus than those of K. So far weve been looking at mz values in a mass spectrum as whole numbers but its possible to get far more accurate results using a high resolution mass spectrometer.
11 8 Fragmentation Patterns In Mass Spectrometry Chemistry Libretexts
Hundreds of research laboratories scattered all over the world use MS every day to investigate fundamental phenomena on the molecular level.
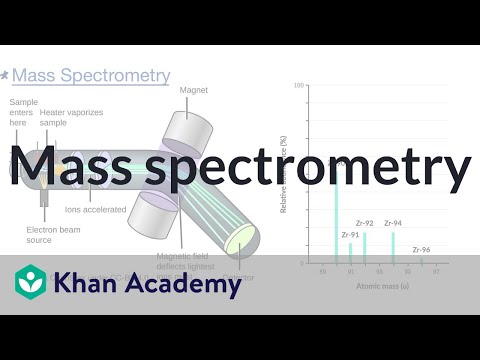
. 1 d Write the ground-state electron configuration of an atom of the element that you identified in part c. You can use that more accurate information about the mass of the molecular ion to work out the molecular formula of the compound. Mass spectrometers are used for finding relative abundances of selected isotopes by ionizing a sample accelerating the sample then as a function of the ions masscharge each bends around the corner interacting with the magnet in characteristic bends which travel to the detector.
ВІ 3 b Describe how to use the information from the mass spectrum to determine the average atomic mass of the element B 1 y Identify the element. A sample of a pure element is analyzed using a mass spectrometer. Most people dont get in a sweat over this and just use the numbers as they are.
It has contributed to numerous discoveries in chemistry physics and biochemistry. For instance we can have. Finally write a ground-state electron configuration of an atom of the element you previously identified above.
Not all ions will hit the detector but the magnetic field strength can be altered to ensure that all the ion streams passing. The most intense ion is assigned an abundance of 100 and it is referred to as the base peak. Connecting with your previous query the mysterious mz 382 could be chosen and made to break into pieces in tandem.
A mass spectrum is an output from a mass spectrometer. The stricter the requirements for a mass spectral match the greater the potential for a false negative result. Mass spectrometry consists basically of weighing ions in the gas.
Mass spectrometry MS is an analytical laboratory technique to separate the components of a sample by their mass and electrical charge. How many different isotopes of the element were in the sample. The mass spectrum is run under a vacuum to prevent the presence of anything other than the ions being tested.
Then you try the tandem approach. Setting restrictions on matching spectra requires replicate studies to measure spectral. BI VE 0 10000 Word Limit b Describe how to use the information from the mass spectrum to determine the average atomic mass of the element.
Amu 24 and percent abundance just below 80. Gas phase reactivity study. Qualitative and quantitative analysis of components in a mixture.
Amu 25 and percent abundance 10. The results are shown below. Mass spectrometry is an analytical technique that involves the study in the gas phase of ionized molecules with the aim of one or more of the following.
The total mass of these 100 typical atoms would be. Using a mass spectrum to find a molecular formula. A sample of a pure element is anylazed using a mass spectrometer.
The Nature of Mass Spectra A mass spectrum will usually be presented as a vertical bar graph in which each bar represents an ion having a specific mass-to-charge ratio mz and the length of the bar indicates the relative abundance of the ion. You take a certain ions from the first MS take it to another one cause fragmentation by various means and use that mass spectrum for final confirmation of the unknown. The average mass of these 100 atoms would be 91318.
Ad Agilent has led innovation and performance in mass spectrometry for over 40 years. Overcome any laboratory challenge. In mass spectrometry a sample containing the atoms or molecules of interest is injected into an instrument called a mass spectrometer.
The results are Shown below. BDescribe how to use the information from the mass spectrum to determine the average atomic mass of the element. Amu 26 and percent abundance just above 10.
The instrument used in MS is called mass spectrometer. The simplest answer to this question is to ask if the measured spectrum matches the reference compound based on a set of requirements for determining a match. Describe how to use the information from the mass spectrum to determine the average atomic mass of the element.
Isotope 1 with atomic mass and relative abundance where is a fraction in the form it gives the percentage of that isotope relative to the total. Most of the ions formed in a mass. Mass spectrometry MS is a mainstream chemical analysis technique in the twenty-first century.
The results are shown below. 515 x 90 112 x 91 171 x 92 174 x 94 28 x 96 91318. Take your mass spectrometry analysis to the next level.
The sampletypically in an aqueous or organic solutionis immediately vaporized by a heater and the vaporized sample is then bombarded by high-energy electrons. It produces a mass spectrum that plots the mass-to-charge mz ratio of compounds in a mixture. How many different isotopes of the element were in the sample.
When we know the mass spectrum of a certan element it means we know the relative abundance of each isotope together with the atomic mass of the isotope. Percent Abundance 24 25 26 a How many different isotopes of the element were in the sample.

Mass Spectra An Overview Sciencedirect Topics
Massenspektrum Molekul Ion Signal Oder Kurzer Molpeak

Mass Spectrometry In Biological Research An Easy Intro

Mass Spectra An Overview Sciencedirect Topics
11 8 Fragmentation Patterns In Mass Spectrometry Chemistry Libretexts

Mass Spectra An Overview Sciencedirect Topics

Mass Spec Basics For The Absolute Novice

Mass Spectrum Of Ethanol Fragmentation Pattern Of Ions For Analysis And Identification Of Ethyl Alcohol Image
0 Comments